
Project Information
Adoption
25 September, 2025
Project Introduction
Real World Data (RWD)
The Real World Data (RWD) project demonstrates how HL7 FHIR can directly support clinical research and regulatory uses by defining FHIR profiles that retrieve research-relevant data from EHR systems. The published Implementation Guide provides a standard, interoperable way for healthcare data to flow into research and regulatory contexts, reducing burden and improving consistency.
What is Real World Data?
Real World Data (RWD) refers to data collected during routine patient care, such as diagnoses, prescriptions, and lab results, rather than in controlled clinical trial settings. When analyzed, RWD can generate Real World Evidence (RWE), offering insights into safety, effectiveness, and patient outcomes that support research and regulatory decision-making.
This project defines a minimal set of FHIR resources and elements that can be consistently retrieved from EHRs for research purposes.
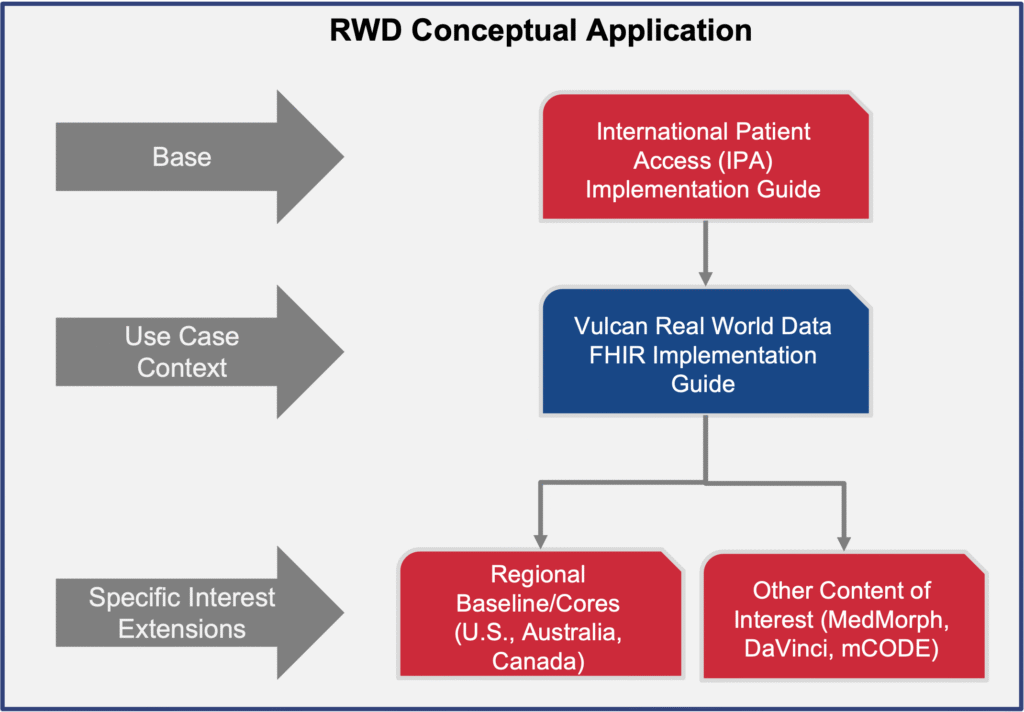
The published Real World Data Implementation Guide (IG):
- Builds on the International Patient Access (IPA) project to ensure broad applicability across jurisdictions.
- Provides a foundation for downstream mappings (e.g., to CDISC or OMOP) while demonstrating how FHIR itself can support clinical research and regulatory needs.
- Defines profiles and search parameters that EHRs should support to enable consistent access to data for retrospective research use cases.
Real World Data (RWD)
Project Status
This status is updated irregularly, please see the project dashboard in the resources below for the latest updates.
- Version 1 of the RWD IG is published and available for adoption.
- The current priority is to expand implementation and gain real-world feedback from adopters.
- Future iterations may extend scope to include registries, payers, HIEs, and prospective use cases (eSource).
Vulcan projects, including RWD, are open to participation. Vulcan is a member-based initiative where anyone with an interest in advancing digital clinical research can join project teams, contribute expertise, and help shape future standards.
Real World Data (RWD)
Project Resources
Project workspace with meeting notes, documentation, and collaboration materials.
Central repository for project artifacts, reference materials, and shared files.
A live view of project progress, milestones, and activities, providing an at-a-glance summary of the project’s current status.
Real World Data (RWD)
Get Involved
The RWD project is now seeking adopters to put the IG into practice. You can:
- Test and implement the published profiles in your EHR or research system
- Share feedback on implementation challenges and opportunities
- Get in touch to explore opportunities for adoption and future releases




